Corrosion Control - Galvanic Tables
Index
- Galvanic Potential Chart
- ASM Handbook, Vol. 13 p. 675.
- Galvanic Table from MIL-STD-889
- Galvanic Voltages relative to Gold
- Galvanic Voltage relative to standard electrode
- When is stainless steel passive or active
- Discussion on Galvanic Table
Note that there are several tables presented on this page and that they do not all agree. These have been collected from various sources and I list them all so you can judge for yourself the variation in the literature. If you are measuring your boat to see if it has the hull potential it should, I suggest you use a zinc reference and look for 0 volts as that is the goal. That leaves aside the calibration problem as zinc to zinc is 0 volts no matter how you define the reference.
Galvanic Potential
The table below reports the Corrosion potentials or Galvanic Series of metals in flowing sea water at ambient temperature.The unshaded symbols show ranges exhibited by stainless steels in acidic water such as may exist in crevices or in stagnant or low velocity or poorly aerated water where Stainless Steel become active, while the shaded areas show the potentials of Stainless Steel when is in passive state.
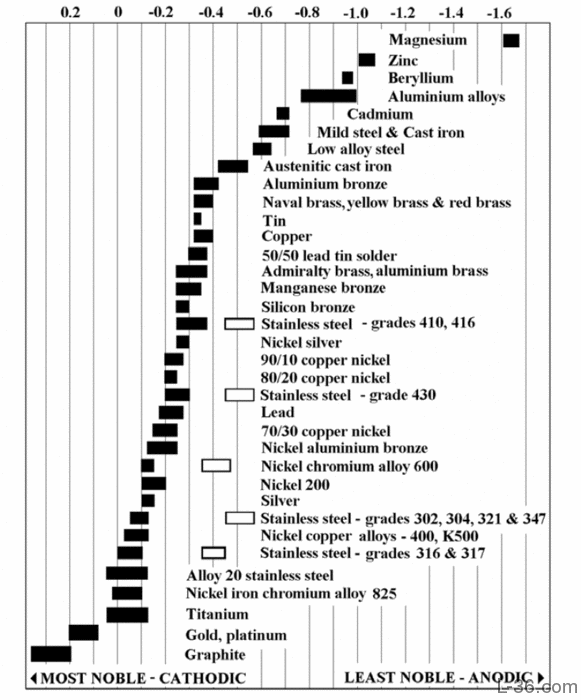
Galvanic Series In Flowing Sea Water
Steady State Electrode Material Potential, Volts referenced to Saturated Calumel Half-Cell| Graphite | +0.25 |
| Platinum | +0.15 |
| Zirconium | 0.04 |
| Type 316 Stainless Steel (Passive) | 0.05 |
| Type 304 Stainless Steel (Passive) | 0.08 |
| Monel 400 | 0.08 |
| Hastelloy C | 0.08 |
| Titanium | 0.1 |
| Silver | 0.13 |
| Type 410 Stainless Steel (Passive) | 0.15 |
| Type 316 Stainless Steel (Active) | 0.18 |
| Nickel | 0.2 |
| Type 430 Stainless Steel (Passive) | 0.22 |
| Copper Alloy 715 (70-30 Cupro-Nickel) | 0.25 |
| Copper Alloy 706 (90-10 Cupro-Nickel) | 0.28 |
| Copper Alloy 443 (Admiralty Brass) | 0.29 |
| G Bronze | 0.31 |
| Copper Alloy 687 (Aluminum Brass) | 0.32 |
| Copper | 0.36 |
| Alloy 464 (Naval Rolled Brass) | 0.4 |
| Type 410 Stainless Steel (Active) | 0.52 |
| Type 304 Stainless Steel (Active) | 0.53 |
| Type 430 Stainless Steel (Active) | 0.57 |
| Carbon Steel | 0.61 |
| Cast Iron | 0.61 |
| Aluminum 3003-H | 0.79 |
| Zinc | 1.03 |
Galvanic Table from MIL-STD-889.
Listed below is the latest galvanic table from MIL-STD-889. For any
combination of dissimilar metals, the metal with the lower number will
act as an anode and will corrode preferentially.
The table is the galvanic series of metals in sea water from Army Missile
Command Report RS-TR-67-11, "Practical Galvanic Series."
The Galvanic Table
Active (Anodic)
- Magnesium
- Mg alloy AZ-31B
- Mg alloy HK-31A
- Zinc (hot-dip, die cast, or plated)
- Beryllium (hot pressed)
- Al 7072 clad on 7075
- Al 2014-T3
- Al 1160-H14
- Al 7079-T6
- Cadmium (plated)
- Uranium
- Al 218 (die cast)
- Al 5052-0
- Al 5052-H12
- Al 5456-0, H353
- Al 5052-H32
- Al 1100-0
- Al 3003-H25
- Al 6061-T6
- Al A360 (die cast)
- Al 7075-T6
- Al 6061-0
- Indium
- Al 2014-0
- Al 2024-T4
- Al 5052-H16
- Tin (plated)
- Stainless steel 430 (active)
- Lead
- Steel 1010
- Iron (cast)
- Stainless steel 410 (active)
- Copper (plated, cast, or wrought)
- Nickel (plated)
- Chromium (Plated)
- Tantalum
- AM350 (active)
- Stainless steel 310 (active)
- Stainless steel 301 (active)
- Stainless steel 304 (active)
- Stainless steel 430 (active)
- Stainless steel 410 (active)
- Stainless steel 17-7PH (active)
- Tungsten
- Niobium (columbium) 1% Zr
- Brass, Yellow, 268
- Uranium 8% Mo.
- Brass, Naval, 464
- Yellow Brass
- Muntz Metal 280
- Brass (plated)
- Nickel-silver (18% Ni)
- Stainless steel 316L (active)
- Bronze 220
- Copper 110
- Red Brass
- Stainless steel 347 (active)
- Molybdenum, Commercial pure
- Copper-nickel 715
- Admiralty brass
- Stainless steel 202 (active)
- Bronze, Phosphor 534 (B-1)
- Monel 400
- Stainless steel 201 (active)
- Carpenter 20 (active)
- Stainless steel 321 (active)
- Stainless steel 316 (active)
- Stainless steel 309 (active)
- Stainless steel 17-7PH (passive)
- Silicone Bronze 655
- Stainless steel 304 (passive)
- Stainless steel 301 (passive)
- Stainless steel 321 (passive)
- Stainless steel 201 (passive)
- Stainless steel 286 (passive)
- Stainless steel 316L (passive)
- AM355 (active)
- Stainless steel 202 (passive)
- Carpenter 20 (passive)
- AM355 (passive)
- A286 (passive)
- Titanium 5A1, 2.5 Sn
- Titanium 13V, 11Cr, 3Al (annealed)
- Titanium 6Al, 4V (solution treated and aged)
- Titanium 6Al, 4V (anneal)
- Titanium 8Mn
- Titanium 13V, 11Cr 3Al (solution heat treated and aged)
- Titanium 75A
- AM350 (passive)
- Silver
- Gold
- Graphite
Galvanic Voltages relative to Gold
To determine the potential of a battery, take the difference between the numbers for the two metals that make up the battery from this table.| Metallurgy | Index (V) |
| Gold, solid and plated, Gold-platinum alloy | 0.00 |
| Rhodium plated on silver-plated copper | 0.05 |
| Silver, solid or plated; monel metal. High nickel-copper alloys | 0.15 |
| Nickel, solid or plated, titanium an s alloys, Monel | 0.30 |
| Copper, solid or plated; low brasses or bronzes; silver solder; German silvery high copper-nickel alloys; nickel-chromium alloys | 0.35 |
| Brass and bronzes | 0.40 |
| High brasses and bronzes | 0.45 |
| 18% chromium type corrosion-resistant steels | 0.50 |
| Chromium plated; tin plated; 12% chromium type corrosion-resistant steels | 0.60 |
| Tin-plate; tin-lead solder | 0.65 |
| Lead, solid or plated; high lead alloys | 0.70 |
| Aluminum, wrought alloys of the 2000 Series | 0.75 |
| Iron, wrought, gray or malleable, plain carbon and low alloy steels | 0.85 |
| Aluminum, wrought alloys other than 2000 Series aluminum, cast alloys of the silicon type | 0.90 |
| Aluminum, cast alloys other than silicon type, cadmium, plated and chromate | 0.95 |
| Hot-dip-zinc plate; galvanized steel | 1.20 |
| Zinc, wrought; zinc-base die-casting alloys; zinc plated | 1.25 |
| Magnesium & magnesium-base alloys, cast or wrought | 1.75 |
| Beryllium | 1.85 |
Galvanic Voltage relative to standard electrode

Short Table
| ANODIC (Least Noble) |
| Magnesium |
| Zinc |
| Aluminium |
| Carbon steel or cast iron |
| Copper alloys (brass, bronze ) |
| Lead |
| STAINLESS STEEL |
| Nickel alloys (Incoloy 825,Hastelloy B) |
| Titanium |
| Graphite |
| CATHODIC (Most Noble) |
When is stainless steel passive or active - formation of the passive layer
The inherent corrosion resistance of stainless steels is derived from alloying the base iron with chromium. BS EN 10088-1 states that a steel must have a minimum of 10.5% (by weight) chromium and a maximum of 1.2% carbon to be classified as 'stainless'. Other alloying elements including nickel, molybdenum, nitrogen, titanium (or niobium) are added to form the various grades. These additions are made to enhance the 'basic' corrosion resistance of the steel but can also usefully modify other properties, such as formability, strength and cryogenic toughness. The corrosion resistance of stainless steel arises from a 'passive', chromium-rich, oxide film that forms naturally on the surface of the steel. Although extremely thin at 1-5 nanometres (i.e. 1-5 x 10-9 metres) thick, this protective film is strongly adherent, and chemically stable (i.e. passive) under conditions which provide sufficient oxygen to the surface. This 'normal' condition is the passive state. The key to the durability of the corrosion resistance of stainless steels is that if the film is damaged it will normally self repair (provided there is sufficient oxygen available). However, under certain conditions, the passive state can be broken down, resulting in corrosive attack.If damaged, the film will normally repair itself. If the film is destroyed the surface is said to be in the active state.Discussion on Galvanic Table (Almost straight from MIL-STD-889)
AC43.13, starting at Par 247, briefly covers several types of corrosion and corrosion protection. The grouping of materials is an early method of MS33586 which was superseded in 1969 by MIL-STD-889.General
The Galvanic Table lists metals in the order of their relative activity in sea water environment. The list begins with the more active (anodic) metal and proceeds down the to the least active (cathodic) metal of the galvanic series.A "galvanic series" applies to a particular electrolyte solution; hence for each specific solution which is expected to be encountered for actual use, a different order or series will ensue. The sea water galvanic series is the most complete series that I know and I have not seen another series published by either the Army, Navy, or Air Force. Civilian aircraft encounter moisture and a salt of some kind.
Galvanic series relationships are useful as a guide for selecting metals to be joined, will help the selection of metals having minimal tendency to interact galvanically, or will indicate the need or degree of protection to be applied to lessen the expected potential interactions.
Generally, the closer one metal is to another in the series, the more compatible they will be, i.e., the galvanic effects will be minimal. Conversely, the farther one metal is from another, the greater the corrosion will be.
Notice that graphite is at the bottom of the table. Think of the corrosion potential if you put a big hunk of graphite on a small piece of magnesium.
In a galvanic couple, the metal higher in the series (or the smaller the number I have given it) represents the anode, and will corrode preferentially in the environment.
Types of Protection
Metals widely separated in the galvanic series must be protected if they are to be joined. Appropriate measures should be taken to avoid contact. This can be accomplished by several methods:- Sacrificial - by applying to the cathodic member a sacrificial coating having a potential similar to or near that of the anodic member. If you are designing for a sacrificial element, the sacrificial element should be on the anodic side and smaller. Cadmium plate (No. 10) on steel bolts (No. 81) holding 2024-T4 (No. 25) plates will sacrifice the cadmium instead of corroding the Aluminum. This is one reason for using new bolts that have the Cad plate intact. (Don't use Cad plate with Titanium (No. 82 through 88). But that's another story.)
- Sealing - by sealing to insure that faying surfaces are water-tight. (We have "talked" about this before.)
- Resistance - by painting or coating all surfaces to increase the resistance of the electrical circuit. (We have "talked" about this only in terms of primer and sealant on fayed surfaces. There is still more that can be done by design selection.)
The (Non-Aerodynamic) Area Rule
To avoid corrosion, avoid a small anodic area relative to the cathodic area.Corollary I - Use LARGE ANODE AREA.
Corollary II - The larger the relative anode area, the lower the galvanic current density on the anode, the lesser the attack.
Corollary III - The amount of galvanic corrosion may be considered as proportional to the Cathode/Anode area ratio.
Corollary IV - Design for a SMALL Cathodic/Anodic Ratio (CAR). (When designing, remember your small CAR.)
Corollary V - The same metal or more noble (cathodic, higher number in the table) metals should be used for small fasteners and bolts.
Sea Water Environments
Metals exposed to sea water environments shall be corrosion and stress corrosion resistant or shall be processed to resist corrosion and stress-corrosion. Irrespective of metals involved, all exposed edges should be sealed with a suitable sealant material conforming to MIL-S-8802. When non-compatible materials are joined, an interposing material compatible with each shall be used.Non-Metallic Materials
Material other than true metals, i.e., non-metallic materials which must be considered as metallic materials, unless there is supporting evidence to the contrary. If these material are essentially free of corrosive agents (salts), free of acid or alkaline materials (neutral pH), and free of carbon or metallic particles, not subject to biodeterioration or will not support fungal growth, and do not absorb or wick water, then these may be considered non-metallics suitable for joining to metals.Many materials classed non-metallic will initiate corrosion of metals to which they are joined, e.g., cellulosic reinforced plastics, carbon or metal loaded resin materials, asbestos-cement composites.
More Precautions for Joining
Where it becomes necessary that relatively incompatible metals must be assembled, the following precautions and joining methods are provided for alleviation of galvanic corrosion.For Electrical Connection - Select materials which are indicated to be more compatible in accordance with the galvanic series.
Design metal couples so that the area of the cathode is smaller (appreciably) than the area of the anodic metal. For example, bolts or screws of stainless steel for fastening aluminum sheet, but not reverse.
Interpose a compatible metallic gasket or washer between the dissimilar metals prior to fastening.
Plate the cathodic member with a metal compatible to the anode.
Select a electrically conductive sealant. (More on these later.)
Not For Electrical Conductors - Interpose a non-absorbing, inert gasket material or washer between the dissimilar materials prior to connecting them.
Other Approaches
Seal all faying edges to preclude the entrance of liquids.Apply corrosion-inhibiting pastes or compounds under heads of screws or bolts inserted into dissimilar metal surfaces whether or not the fasteners had been previously plated or otherwise treated. In some instances, it may be feasible to apply an organic coating to the faying surfaces prior to assembly. This would be applicable to joints which are not required to be electrically conductive.
Where practicable or where it will not interfere with the proposed use
of the assembly, the external joint should be coated externally with an
effective paint system.
NOTICE: Some pages have affiliate links to Amazon. As an Amazon Associate, I earn from qualifying purchases. Please read website Cookie, Privacy, and Disclamers by clicking HERE. To contact me click HERE. For my YouTube page click HERE